The Principle and Method of Western Blotting (WB)
Contact us for help in setting up your experiments
The principle
In Western blotting (WB), target proteins are transferred to a hydrophobic membrane after SDS-PAGE and detected using specific antibodies.
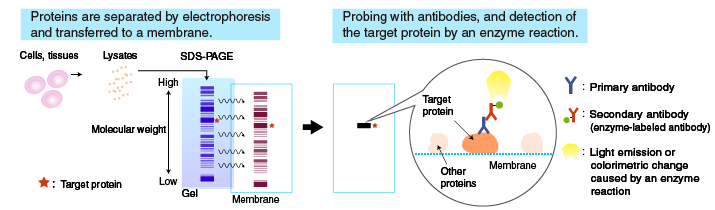
After SDS-PAGE, a membrane is placed on the gel, to which the separated proteins in the gel are electrophoretically transferred. The membrane with transferred proteins is then probed with a primary antibody (an antibody specific for the target protein), washed, and reacted with a secondary antibody labeled with an enzyme, such as horseradish peroxidase (HRP). The bound enzyme activity is used to detect the target protein and visualized by a chemiluminescent or chromogenic method.
The following sections describe the procedure starting from electrotransfer of proteins to the membrane.
Follow the links below for the methods used to generate antibodies, and the principle and method of SDS-PAGE.
- How to generate antibodies
- The principle and method of polyacrylamide gel electrophoresis (SDS-PAGE)
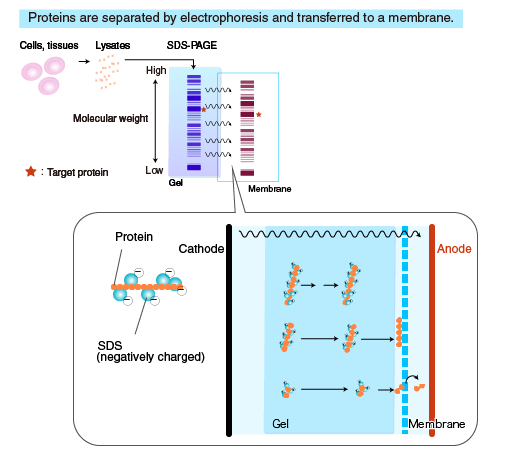
Transfer to membrane
Proteins separated by SDS-PAGE are “transferred” from the polyacrylamide gel to a membrane, using a specialized apparatus (blotting apparatus). A semi-dry or a tank system can be used for transfer.
Comparison between blotting apparatuses
| Semi-dry System | Tank System | |
|---|---|---|
| Blotting Time | Short | Long |
| Buffer Volume | Small | Large |
| Transfer Efficiency | Poor for high molecular weight proteins | Good for high molecular proteins Evenly transferred |
Membrane
Nitrocellulose and PVDF membranes are commonly used. Though more expensive, the PVDF membrane is stronger and has a high absorption capacity with proteins, which makes it suitable for CBB staining, detection with certain substrates for alkaline phosphatase, and re-probing. Nitrocellulose membrane is more fragile but less expensive than PVDF, and does not require pre-wetting with methanol or cause as much non-specific binding.
Re-probing: Stripping the membrane of antibodies after detection, and probing with another antibody.
Transfer buffer
Different buffers are used for transfer, depending on the molecular weight and the nature of the target proteins. The composition of a commonly used transfer buffer is shown below.
Composition of transfer buffer (Commonly used)
25 mM Tris-HCI
192 mM glycine
20% MeOH
Methanol stabilizes the gel and prevents gel from swelling. When transferring high molecular weight proteins, reduce the methanol concentration to allow the gel to swell slightly, which facilitates protein transfer.
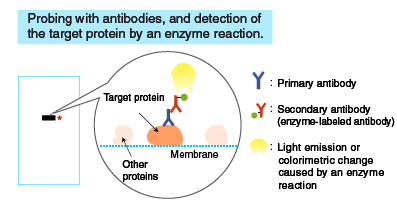
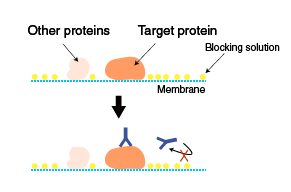
Blocking and probing with antibodies
Blocking
The membrane with transferred proteins is blocked to prevent non-specific binding of antibodies to the membrane. Blocking only prevents non-specific binding, and does not reduce cross-reactivity (specific binding of antibodies to non-target proteins containing the same epitope as found on the target protein). Various solutions are used for blocking. Commonly used blocking solutions are described below.
1-3% BSA in PBS-T
Bovine serum albumin (BSA) is used as a blocking agent.
1-5% skim milk in PBS-
Skim milk is more effective in blocking and used frequently. However, it can interfere with specific antigen-antibody reactions.
Skim milk contains the phosphoprotein casein, and therefore is not suitable for the detection of phosphoproteins.
PBS-T (PBS-Tween 20): Phosphate-buffered saline (PBS) containing 0.05% Tween 20
Probing with antibodies
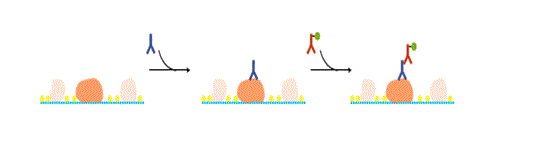
Following blocking, the membrane is probed with a primary antibody, and then with a secondary antibody. The concentration of the primary antibody is critical. If it’s too low, the target protein cannot be detected. If it’s too high, non-specific reactions occur, resulting in detection of non-target proteins.
Determining an optimal concentration of the primary antibody
When using an antibody for the first time, choose a concentration based on the literature or stated on the product datasheet. If desired results are not obtained, test several different dilutions of the primary antibody, or try a different dilution buffer.
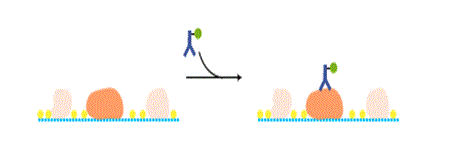
Secondary antibody
Commercially available HRP- or alkaline phosphatase (AP)-labeled secondary antibodies are commonly used. Check the isotype of the primary antibody and the animal species in which the primary antibody is raised. Care should be taken to avoid using an unsuitable secondary antibody.
Instead of a secondary antibody, an HRP-labeled primary antibody can be used to reduce the processing time. It also eliminates non-specific signals caused by non-specific binding of a secondary antibody.
Related Products: HRP-DirecT series
Detection of bands
A reaction mixture containing a substrate is added to the membrane. The enzyme attached to the antibody catalyzes a reaction that emits light, which is detected by X-ray film or a cooled CCD camera.
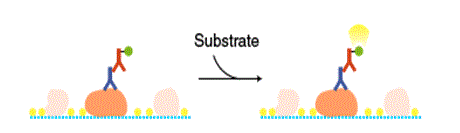
Colormetric method
A chromogenic substrate is used as a detection reagent. Diaminobenzidine (DAB) or TMB are commonly used as substrates of HRP, and a mixture of BCIP and NBT is used as a substrate of alkaline phosphatase (AP).
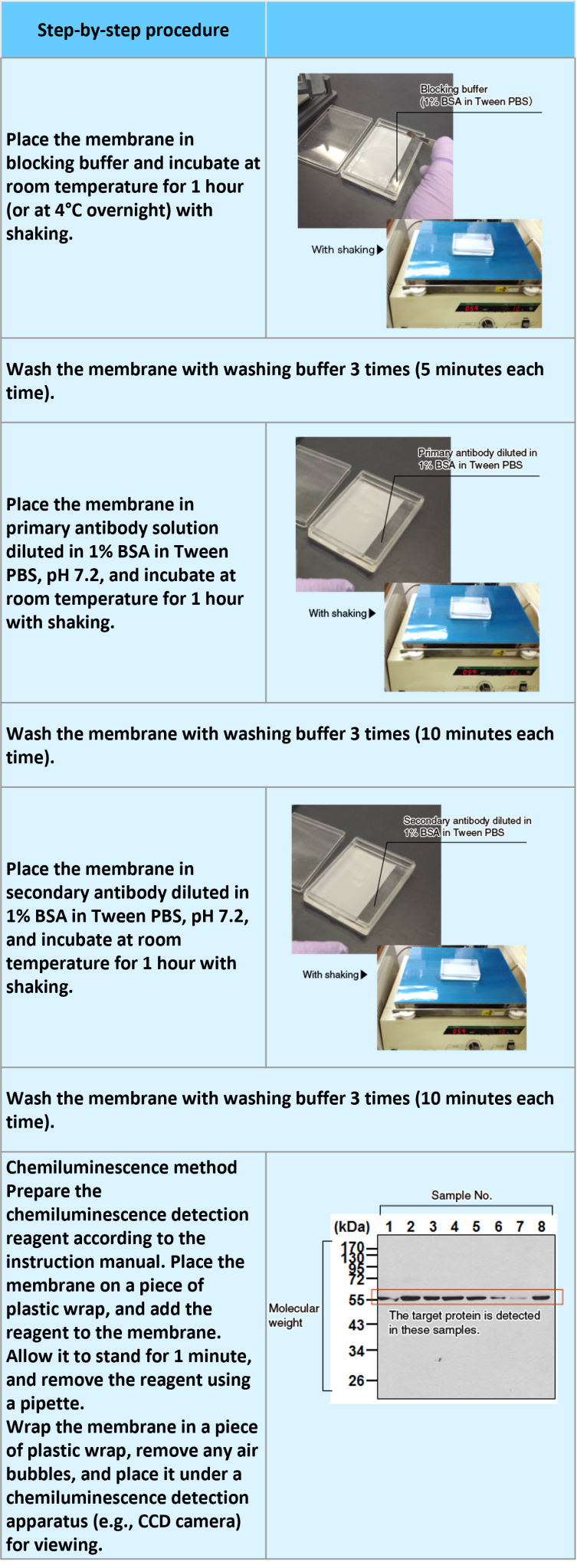
Procedure
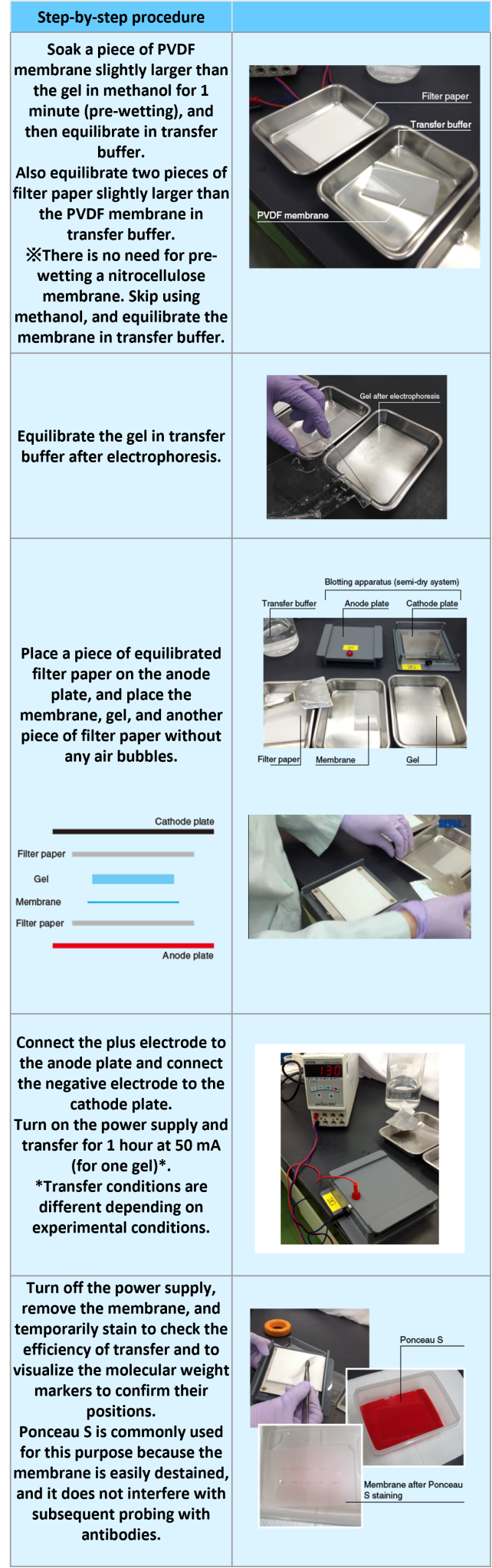
Transfer of proteins to a membrane (semi-dry type blotting and membrane staining)
An example performed at MBL
Probing with antibodies and detection of the bands (blocking and detection of the antigen by HRP reaction)
An example performed at MBL
Links to Western blotting (WB)-related products
| Primary antibodies suitable for Western blotting (WB) (excluding anti-tag antibodies) | List of monoclonal primary antibodies List of polyclonal primary antibodies |
| Anti-tag antibodies suitable for Western blotting (WB) | List of monoclonal antibodies for tags List of polyclonal antibodies for tags |
| Control antibodies | List of antibodies for loading control |
| For shorter processing time! HRP direct-labeled antibodies |
These antibodies are high-sensitivity, HRP-labeled antibodies requiring a reaction time as short as 30 minutes. Non-specific signals caused by secondary antibodies are also eliminated. No need for a secondary antibody! HRP DirecT series |
Autophagy Pathway
Autophagy is a cellular self-digestion process for the purpose of providing nutrients to allow for cell survival during stress conditions.
Pathway Posters
View all Pathway Posters
Related Links
Antibody
Antibody basics
Antibodies as a research tool
- How to generate antibodies
- How to select antibodies
- Labeled antibodies
- How to label antibodies
- Main causes of non-specific reactions
- How to reduce non-specific reactions
- Tags and Tag antibodies
Qualitative and quantitative measurements of proteins using antibodies
- Western blotting (WB)
- Enzyme-linked Immunosorbent assay (ELISA)
- Immunoprecipitation (IP)
- Co-immunoprecipitation (Co-IP)

