How to generate antibodies
How to generate antibodies by immunizing animals with an antigen (immunogen)
How to generate antibodies without immunizing animals
Related topics
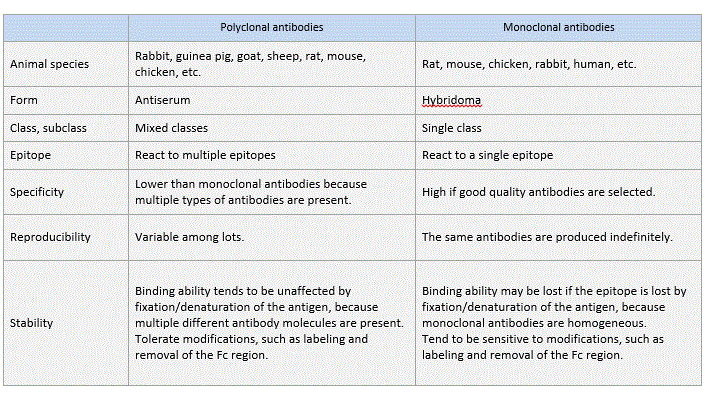
Polyclonal antibodies
An antigen (immunogen) injected into animals induces them to produce and secrete high levels of antibodies into the blood. Several months after repeated immunization, the blood (plasma, serum) is collected, and antibodies are purified (by methods described later). The antibodies generated by this method are called polyclonal antibodies because they are derived from different B cell clones and the resulting antiserum contains numerous different antibodies that react to the injected immunogen.
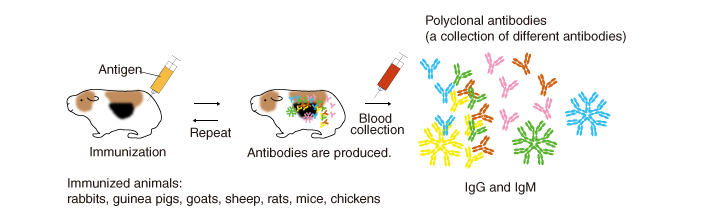
Animals used for immunization (immunized animals)
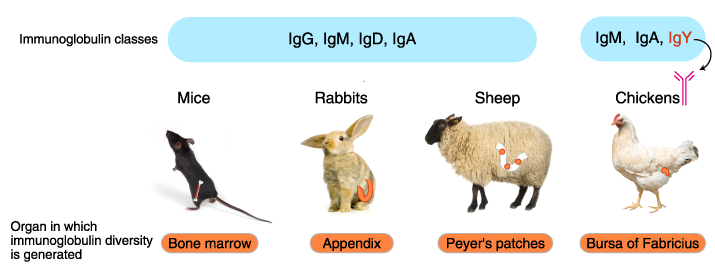
In addition to mice and rabbits, various mammals and birds are used for immunization, including rats, hamsters, guinea pigs, chickens, goats, sheep, and donkeys. Immunoglobulin isotypes, organization of immunoglobulin genes, mechanism of diversification, and the organ sites of antibody diversification differ between vertebrate animals.
How antibodies are produced
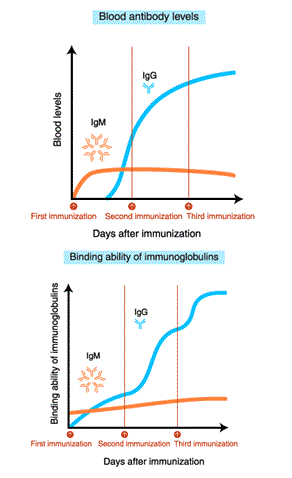 After immunization, blood IgM levels increase first. With repeated immunization, IgG levels increase.
After immunization, blood IgM levels increase first. With repeated immunization, IgG levels increase.
The affinity (binding ability) of IgM for the antigen does not increase even after repeated immunization. In contrast, the binding ability of IgG increases with repeated immunization.
Dangers of hyperimmunization!
Extending the immunization period, in the hopes of developing a higher affinity antibody to the antigen, is not necessarily beneficial. After an excessively long immunization period, non-specific antibodies, including self-reactive antibodies, increase in amount and the animals’ health may deteriorate. To generate high quality antibodies, it is important to periodically collect a small amount of blood and evaluate the affinity of the antibody for the antigen and non-specific reactions.
Monoclonal antibodies
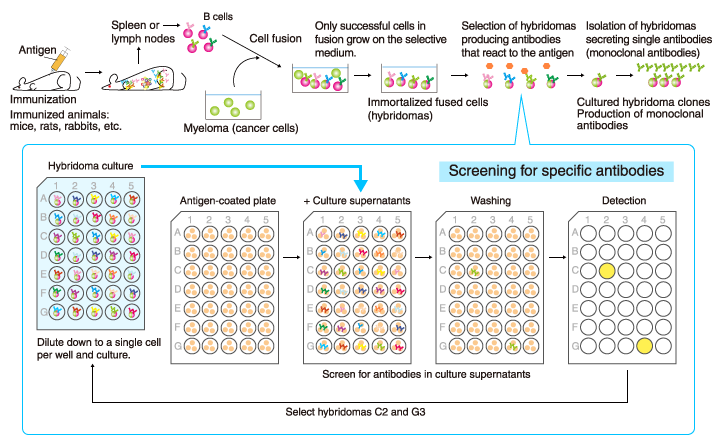
A single antibody (monoclonal antibody) can be stably produced if a single B cell producing the antibody is isolated and cultured indefinitely. This is achieved by artificially fusing the antibody-producing B cells with immortalized cancer cells (myeloma) to generate hybridomas that live indefinitely and contain genes encoding specific antibodies, and by selecting the hybridoma clones that produce the desired monoclonal antibodies with high affinity and specificity.
It usually takes 4-6 months from immunization of animals to production of monoclonal antibodies.
Difference between clones in monoclonal antibodies
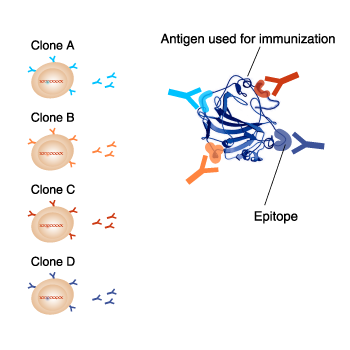 Even though monoclonal antibodies against the same antigen are produced, each clone reacts to different epitopes on the same antigen. Also, each clone can have different suitable applications. It is important to take note of the antigen name and clone name when selecting a monoclonal antibody.
Even though monoclonal antibodies against the same antigen are produced, each clone reacts to different epitopes on the same antigen. Also, each clone can have different suitable applications. It is important to take note of the antigen name and clone name when selecting a monoclonal antibody.
Phage display method
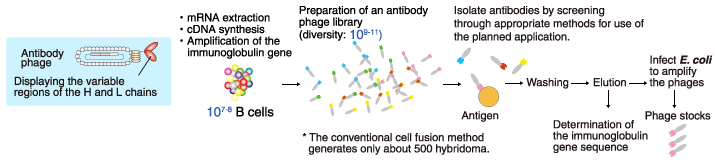
A phage (bacteriophage) is a virus that infects bacteria. The phage genetically integrated with genes, coding a part of the antibody, is referred to as an antibody phage, and displays on the surface of the antibody fragment, such as Fab form or scFv form. An antibody phage library is a huge mixed population of antibody phages that can react with various molecules. In the phage display method, antibodies against the target molecule are selected from the antibody phage library by using a molecular affinity of antibodies and the target molecule.
Various sources of the immunoglobulin gene are used in the antibody phage library. For example, naïve antibody phage libraries are prepared using the B cells of healthy individuals. Other examples include B cells from patients whose sera contain antibodies that potently neutralize pathogens or toxins, and B cells from animals immunized with a specific antigen.
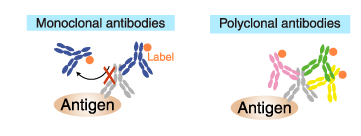
Difference between polyclonal and monoclonal antibodies
Antigen binding
An antigen binding monoclonal antibody reacts to a single epitope. Therefore, only one antibody molecule can bind to an antigen molecule.
In contrast, a polyclonal antibody is a collection of immunoglobulin molecules that react against a specific antigen and each recognizes a different epitope. Therefore, multiple antibody molecules bind to an antigen molecule. (if succiently large).
Labeled (secondary) antibodies
Similarly, only one antibody molecule can bind to a primary antibody molecule if the secondary antibody is a labeled monoclonal antibody. Whereas multiple antibodies can bind to a primary antibody molecule if the secondary antibody is a polyclonal antibody.
Consequently, polyclonal antibodies provide a higher sensitivity of detection (amplification of the signal) and, therefore, are commonly used as a secondary antibody.
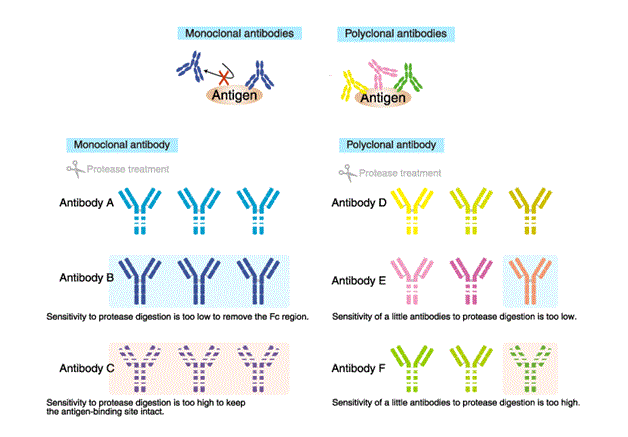
Sensitivity to protein-degrading enzyme (Proteases)
Proteases are often used for antibody modification in order to add a label, or to remove the Fc region to reduce non-specific reaction.
The high sensitivity of monoclonal antibodies to protease digestion can result in digestion outside the intended regions, or loss of antigen-binding ability. Conversely, if the antibody is resistant to the protease, intended modifications would be difficult.
In contrast, sensitivity to proteases is unlikely to cause a problem with polyclonal antibodies, and antigen-binding ability tends to be unaffected because multiple different antibody molecules are present. Thus, polyclonal antibodies are amenable to modification.
How to purify antibodies
Antibodies are usually purified by the following three steps.
- Partially remove solid materials and proteins other than the antibodies. Perform centrifugation or filtration.
- Isolate antibodies by affinity chromatography.
- Remove contaminants remaining after Step 2.
Purification with protein A/G
IgG can be purified using a column packed with immobilized Protein A or Protein G.
Protein A is a cell wall protein of Staphylococcus aureus that specifically binds to the Fc region of mammalian IgG. Staphylococcus aureus is a commensal bacterium of the skin and the gastrointestinal tract of humans and other animals, and occasionally causes an infectious disease or food poisoning. Protein A captures IgG, allowing the bacteria to evade elimination by the immune system.
Protein G: A cell wall protein isolated from group G Streptococci
The ability of Protein G and Protein A to bind to Ig differs between Ig subclasses and between species. The use of Protein G or A should depend on the purpose. IgM and IgY are purified by different columns.
Antigen-affinity purification
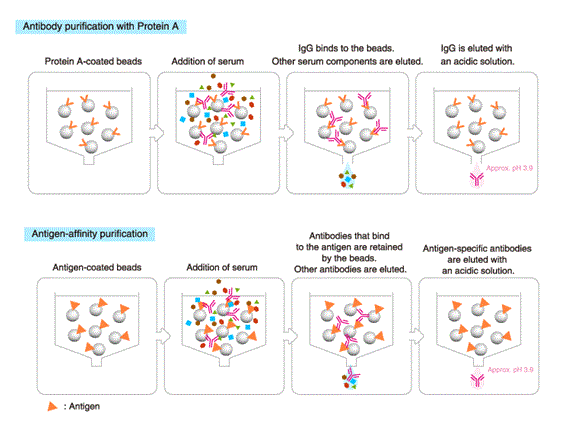
This method provides a higher yield of antigen-specific antibodies than Protein A/G affinity chromatography, though the total amount of recovered antibodies is lower.
Protein A/G affinity chromatography and antigen-affinity chromatography cannot be used if the antibodies are inactivated in an acidic solution. In that case, antibodies are purified by ammonium sulfate precipitation (salting out) or ion exchange chromatography.
- Details of antibody isotypes and subclasses
- Details of affinity chromatography
- Details of gel-filtration chromatography
Related Links
*Antibody
→Antibody basics
→Antibodies as a research tool
- How to select antibodies
- Labeled antibodies
- How to label antibodies
- Main causes of non-specific reactions
- How to reduce non-specific reactions
- Tags and Tag antibodies
*Qualitative and quantitative measurements of proteins using antibodies
- Western blotting (WB)
- Enzyme-linked Immunosorbent assay (ELISA)
- Immunoprecipitation (IP)
- Co-immunoprecipitation (Co-IP)