FAQs – Immune Monitoring
- General
- Product Info/Ordering
- Optimization/Troubleshooting
- CD1d
- MHC Monomers
- QuickSwitch™
What are MHC tetramers?
MHC tetramers are complexes of four major histocompatibility complex (MHC) molecules, which are associated with a specific peptide and bound to a fluorochrome-conjugated streptavidin. There are two types of tetramers, class I and class II. Class I tetramers bind to a distinct set of T cell receptors (TCRs) on a subset of CD8+ T cells. Class II tetramers bind to a distinct population of CD4+ T cells. Thus, by mixing tetramers with peripheral blood mononuclear cells (PBMCs) or whole blood and using flow cytometry as a detection system, all CD4+ or CD8+ T cells that are specific for one peptide in the context of a particular MHC allele can be detected, regardless of functionality. Simply stated, MHC tetramer reagents allow measurement of a cellular immune response directed toward single peptide specificity. The heavy chain portion of human and macaque class I tetramers contains a patented mutation to minimize non-specific binding of tetramers to CD8 on the cell surface, improving the specificity of these reagents to accurately discriminate the rare, antigen-specific T cells from the general CD8+ T cell population.
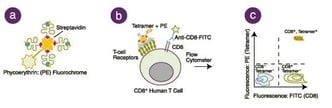
How are MHC tetramers used for research and immune monitoring?
MHC tetramers are used for the detection and monitoring of antigen-specific T cells to study the adaptive immune response, including T cell development; disease progression; and evaluation of therapeutics, vaccinations, and other interventions that may have an impact on cellular immune responses. In clinical trials, MHC tetramers are used to monitor T cells that may be related to either the cause of illness (e.g. diabetes or autoimmune disease) or the fight against an ailment (e.g. cancer, infection). In this way, antigen-specific T cells and their phenotype/function may serve as a surrogate or biomarker that correlates to treatment efficacy or disease progression.
What are some examples of diseases and models that have been studied using MHC tetramers?
- Infectious Diseases: HIV, EBV, CMV, HPV, HBV, HCV, Influenza, Measles, Malaria, TB, RSV
- Cancer: Breast, Prostate, Melanoma, Colon, Lung, Cervical, Ovarian, Leukemia
- Autoimmune Diseases: Diabetes, Multiple sclerosis, Rheumatoid arthritis, Autoimmune vitiligo
- Transplantation: EBV and CMV
- Animal Models: OVA, E alpha, SIV
What are the advantages of MHC tetramer analysis in measuring cellular immune response?
Tetramer analysis offers many potential advantages over some of the more traditional T cell based assays:
- MHC tetramers allow direct detection of antigen-specific T cells, independent of cell function.
- MHC tetramer staining is quantitative.
- MHC tetramer staining does not require the use of radioactive isotopes.
- Staining is rapid, allowing fresh blood or tissue-derived samples to be analyzed. Specific T cells can be detected in blood samples without the need for in vitro culture.
- Large numbers of samples can be processed using a flow cytometer, making tetramer analysis more high throughput than other T cell detection methods. • Cells can be labeled with MHC tetramer and other cell-surface markers at the same time allowing additional characterization of the responding cells.
- MHC tetramer-labeled cells remain viable and can be sorted by flow cytometry for further study, including functional assays.
- MHC tetramers can be used in conjunction with intracellular staining (e.g. for cytokines such as IFN-γ) and other flow cytometry techniques to further characterize the antigen-specific T cell functionality and compare them with T cells that are not antigen-specific.
- Because MHC tetramers are available in different fluorochromes, multiple T cell specificities can be analyzed simultaneously.
How do I choose the peptide and allele for my MHC tetramer studies?
We recommend customers do a literature search to determine the best peptide/allele combination to detect the specific T cells of interest.
Which fluorochromes are available for MHC tetramers?
Most MHC tetramers are offered in PE, APC, and BV421. FITC is offered on a subset of Class I tetramers.
Can tetramers be used on CyTOF?
Metal conjugated tetramers for use on CyTOF instruments have been produced by individual investigators using MHC monomers and streptavidin or neutravidin reagents that are tagged with rare metals. A poster presented at AAI on CyTOF tetramers made with MBLI MHC monomers and a neutravidin reagent to be commercialized by DVS Sciences can be found on our website.
What is the concentration of tetramer in the vial?
We do not specify a tetramer concentration (1 vial = 50 tests), as the fluorochrome contribution can vary in the final formulation. A more accurate and consistent monomer concentration in the vial can be obtained by calling MBLI (1-800-200-5459) with your product code and lot number.
What allele/peptide comprises the Negative Tetramer?
The “Negative Tetramer” is prepared with the HLA-A*02:01 allele and a proprietary peptide whose sequence does not occur in nature. We offer the “Negative Tetramer” conjugated to either PE (PN T01044), APC (PN T01054), and BV421 (PN T01044B).
What is the α3 mutation?
MBL has obtained an exclusive license to utilize a patented class I heavy chain mutated in the α3 domain in the manufacture of human and macaque MHC tetramers. This mutation greatly diminishes nonspecific MHC binding to CD8, but peptide-specific/MHC binding to TCR is retained. Additional information can be found in Bodinier M. et al. Efficient detection and immunomagnetic sorting of specific T cells using multimers of MHC class I and peptide with reduced CD8 binding. Nat Med 2000, 6(6): 707-710.
What is iTAg™?
iTAg™ is a trademark carried on all tetramer products originally developed at Beckman Coulter. In 2013, MBL licensed Beckman Coulter’s entire portfolio of Premium, Select, and Custom MHC tetramers. These products are now manufactured and tested by MBL according to the standards set by Beckman Coulter. The iTAg branding will eventually be phased out, however high standards for manufacturing and QC will remain the backbone of tetramer products offered by MBL International.
What do “Premium,” “Select,” “Custom,” and “T-Select” designations mean?
Premium tetramers are the most commonly ordered PE-labeled tetramers and can be shipped immediately. Select tetramers are PE-, APC- or BV421-labeled tetramers that may need to be assembled from existing monomer stocks. Custom tetramers are manufactured with peptide and allele combinations selected by the customer. While all three classifications of tetramers are all manufactured using the same methods, only Premium tetramers come with a stability claim, which is a minimum of 12 months from the date of manufacture.
How do you purify the MHC complexes?
There are multiple purification steps including centrifugation, dialysis, and chromatography. MHC class I and II molecules are affinity-purified via a biotinylated residue in the MHC complex. In some cases, additional affinity chromatography and size exclusion chromatography steps are performed.
How are the products QC’d?
Steps in the tetramer manufacturing process are verified by spectrophotometry and HPLC. Some tetramers are further tested for functionality.
Do you check for functionality?
We have a type of tetramer called “Premium.” In addition to spectrophotometry and HPLC analyses for QC, “Premium” tetramers are tested by flow cytometry.
What is the difference between paraformaldehyde and formaldehyde?
Paraformaldehyde is a form of formaldehyde.
The simple chemical formula for formaldehyde is CH2O.
Paraformaldehyde is polymerized formaldehyde. When paraformaldehyde is dissolved, it becomes formaldehyde. Only the dissolved formaldehyde form is able to fix tissues.
When you are making a paraformaldehyde solution, you should make it in a hood. Mix paraformaldehyde with PBS or TBS at 70°C. Use 5N NaOH to make the solution clear. You can then do a quick spin or use a filter syringe to remove any insoluble impurities. It’s always best to use a fresh solution but aliquots can be stored at -20°C and used over a couple months.
Can I use biotinylated antibodies or streptavidin conjugates with MHC tetramers and monomers?
It is not recommended to use biotinylated antibodies and streptavidin conjugates in parallel with MHC tetramers and particularly with biotinylated monomers as they could cause non specific staining reactions.
What MHC tetramer species and alleles are available?
The list of alleles available is continuously expanding and currently include Human, Mouse, Rhesus Macaque, Mauritian Cynomolgus Macaque, Chicken, and Human-Mouse Chimera species for class I, as well as Human and Mouse for class II. Our current allele list can be found on the MBLI website and you can search for allele of interest using the tetramer search tool.
What if I don’t see the allele I want on the website?
While we typically cannot prepare custom alleles not listed, new alleles are often in development. In some instances an allele proposed by a customer may be taken on as a new development project. If you are interested in an allele that is not listed, please contact your local distributor or MBL directly (1-800-200-5459) with your inquiry.
How do I order a custom MHC tetramer?
We have a wide selection of class I (multiple species) and class II (human) alleles available for custom tetramer synthesis. You designate the peptide/allele combination of interest based on the literature or your own studies on a custom order request form. Our tetramer R&D manufacturing team will assess the theoretical feasibility (e.g. likelihood of successful tetramer manufacture) of that combination at no charge. Should the team conclude that the tetramer is feasible, you will receive a quote with the price of the tetramer, as well as a projected lead-time.
What happens if the attempt at making my MHC custom tetramer fails?
In the rare event that a custom tetramer is attempted, but the synthesis is not successful due to instability, a feasibility fee is charged to cover the labor and materials for the manufacturing attempt. Additional information can be found in the Monomer/Tetramer Terms and Conditions.
Can you make a custom MHC class II tetramer?
Custom class II tetramers are available for several human class II alleles. Customs are generally not available for mouse class II, as we use a tethered peptide technology for greater tetramer stability. To create a mouse custom, the allele and linked peptide would need to be created from scratch. While this is not impossible, the development costs are significant. If an investigator has a pressing need for a new mouse class II, they would have the option of paying for the development of that new tetramer. If you are interested pursuing a new class II mouse allele that is not listed, please contact your local distributor or MBL directly (1-800-200-5459) with your inquiry.
Can I provide the peptide?
Yes, if it meets our requirements for purity and quantity. We require a minimum of 20 mgs for HLA-“A” and MHC class II alleles or 50 mgs for HLA-“B” alleles. Peptide purity must be greater than or equal to 90%. The Certificate of Analysis for the peptide must be shipped with the peptide.
Alternatively, MBLI can provide the peptide for you for an additional “peptide acquisition fee.” Contact your MBL International sales representative or customer service (1-800-200-5459) for details.
Can I get the leftover peptide back?
Yes, but this request must be made at the time you place your order. The remaining quantity of peptide may be only 0-2 mgs.
How long before I receive product?
Once your order is booked and a purchase order is received, you will be quoted a ship date by customer service. Delivery time is contingent on the type of tetramer you request.
Premium: 1-3 days.
Select or T-Select: 3-30 days. Shipped next day if we have the tetramer, 2-4 weeks if we have the monomer but have to prepare the tetramer.
Custom: Up to 60 days if peptide has to be ordered/synthesized. Can be produced in 3-4 weeks if we have peptide on hand.
How should MHC tetramers be stored?
Tetramers should be stored at 2-8°C. They should not be frozen, and exposure to light should be minimized. Vials should be refrigerated at 2-8°C immediately after use.
What is the shelf life of MHC tetramer reagents?
Some MHC tetramer products have expiration dating, ranging from 12-24 months from the date of manufacture, and for at least 90 days after the vial has been opened, when properly stored at 2-8°C protected from light. Those products will have an expiration date on the vial label. Stability data is not available for other stocked and custom MHC tetramers, but they are manufactured the same as those with expiration dating
Useful Publication for Optimized Staining
“… However, the threshold of TCR affinity amenable to detection with pMHC tetramers with the optimal protocol used here is very low and below that found on the vast majority of cognate T-cells. We also find that an optimal staining with pMHC tetramer can recover cells that cannot be detected using standard pMHC dextramer protocols as currently listed on the Immudex website (March 2018). We regularly see T-cells that can be stained with optimized pMHC tetramer staining that cannot be stained by standard pMHC dextramer staining in the absence of the PKI + Ab; this threshold is graphically depicted in Figure 1. The staining of the dextramer-sorted insulin-β line in Figure 6 provides a good example of these sensitivities; …”
Dolton, G., Zervoudi, E., Rius, C., Wall, A., Thomas, H. L., Fuller, A., . . . Sewell, A. K. (2018). Optimized Peptide–MHC Multimer Protocols for Detection and Isolation of Autoimmune T-Cells. Frontiers in Immunology, 9. doi:10.3389/fimmu.2018.01378
What is the recommended cell labeling procedure?
Protocols for whole blood and mononuclear cell preparations are included on the datasheet that accompanies the product. Datasheets can also be found on the MBLI website.
What lyse and fixative reagents are recommended?
Commercial red blood cell lysing reagents such as VersaLyse™ (Beckman Coulter PN A09777) have been shown to be compatible with tetramer whole blood staining protocols. It is important to read the manufacturer’s recommendations with such reagents to be certain that a suitable anticoagulant is used during blood collection. Methanol-free formaldehyde fixative solutions (e.g. IOTest®3 X10 solution from Beckman Coulter, PN A07800) can be used with tetramers, as indicated in the tetramer staining protocols. Use caution if fixing cells prior to tetramer staining, as some tetramers will not bind post-fixation.
Can I stain with tetramer and antibodies simultaneously?
Yes. For simple 2, 3 or 4 color assays using antibodies conjugated to FITC, PE, APC, PC5, or PC7, you should have no problems. More complex staining experiments (ex. 6, 8, 10, or more colors) may require titration of reagents and/or reagent order of addition studies to optimize staining results. For mouse studies, sequential addition (tetramer first, antibody second) may be helpful if the problematic CD8 clone 53-6.7 is used.
What tetramer should I use as a negative control?
For Class I HLA-A*02:01 tetramers, the MHC Negative Tetramer can be used. Some investigators use the Negative Tetramer as a control for other human alleles but a tetramer of the same allele as the tetramer of interest, built with a non-specific peptide, would be best. This applies to non-human alleles especially. For Class II DR alleles, tetramers made using the human CLIP peptide or a non-specific peptide can serve as negative controls.
What can be done about high background staining?
MHC tetramer labeling may need to be optimized in your particular system. Tetramer staining best practices may include any or all of the following:
- Proper titration of Abs/multicolor panel optimization (PMT balance, minimal spillover, etc.)
- Proper staining temperature: RT (class I) or 37°C (class II), NOT 4°C or ice
- Use of a singlet gate on the flow cytometer to eliminate doublets and aggregates
- Use of viability dyes, particularly when using thawed cells
- Use of dump channels for exclusion of unwanted cell types (B, NK, monocytes, etc.)
- Use of positive gating (e.g. CD3)
- Use an Fc receptor block (e.g. Clear Back) when appropriate
Which CD8 clone should I use?
Anti-human CD8 clone SFCI21Thy2D3 (“T8”) and anti-mouse CD8 clone KT15 are recommended.
Do CD8 antibodies affect the binding of tetramer to specific CD8 T cells?
The α3 mutation that significantly reduces the binding of MHC tetramer to human and macaque CD8 also minimizes the aberrant effect that some CD8 antibodies have on the specific binding of MHC Class I tetramer. Therefore, many different anti-human CD8 antibody clones are compatible with tetramers. Clone SFCI21Thy2D3 (also known as “T8”) is recommended. For experiments on murine cells, the CD8 clone choice is more critical in that clone 53-6.7 should be avoided if possible.
How do I reduce background in my mouse tetramer experiment?
Anti-mouse CD8 clone choice is critical when using H-2 Kb tetramers, as some clones, including 53-6.7, lead to high background staining of all CD8+ T cells, rather than the antigen-specific ones. For best results, clone KT15, available from MBL International in FITC (K0227-4), is recommended. Additional information can be found in the datasheet that accompanies mouse class I tetramers, as well as the following reference containing a protocol for performing a cross titration series for optimization: Current Protocols in Immunology, MHC-Peptide tetramers to Visualize Antigen-Specific T Cells, Section 17.3.23-24, Supplement 53.
What are the recommendations for negative and positive controls?
For negative controls, an irrelevant tetramer and/or a negative target cell may be suitable. Please see the blog post “Tetramer Tips for Success: How to be a (Negative) Control Freak” for a summary of negative control options. For positive controls, cell lines or PBMCs containing cells expressing the specific T cell receptor of interest can be used. Further detail can be found in the blog post “Be positive! 5 ways to confirm your MHC tetramer is binding”.
Which murine allele should be used with C57BL/6 or BALB/c mice?
C57BL/6 mice express H-2 Kb and H-2 Db alleles. BALB/c mice express H-2 Kd, H-2 Dd, and H-2 Ld alleles.
Which blood collection tubes are compatible with tetramers?
EDTA, Lithium Heparin, and Sodium Heparin tubes are all compatible with the tetramer staining protocol. However, some of these anticoagulants (e.g. Lithium Heparin) are NOT compatible with particular lysing buffers such as VersaLyse™. Be sure to check the manufacturer’s recommended protocols for reagent compatibility.
Can I use a biotinylated antibody as part of my staining protocol?
Possibly, but note that there is a small quantity of free biotinylated monomer in the tetramer preparation. This should be considered when planning your experiment.
Can tetramers be used to stain tissue sections?
Yes, tetramer staining of tissue sections has been described in the literature:
Coppieters KT et al. J Exp Med 2012, 209(1): 51-60.
Hong JJ et al. PloS one 2009, 4(1): e4131.
Kawabata T et al. J Med Virol 2012, 84(7): 1120-1127.
Kuon W et al.. J Immunol 2004, 173: 4859-4866.
Skinner PJ et al. J Immunol 2000, 165: 613-617.
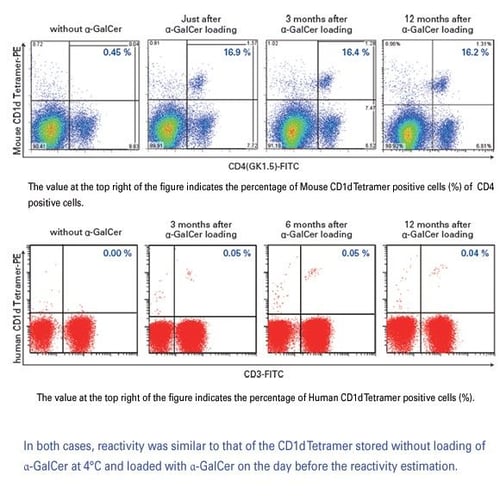
Can I dissolve α-GalCer in DMSO instead of pyridine?
Pyridine is recommended, as it has been shown that tetramer staining can be lost when the tetramer is loaded with α-GalCer dissolved in DMSO.
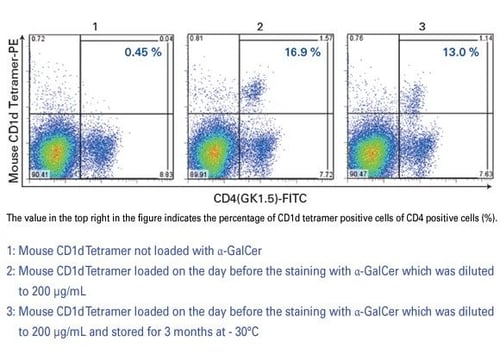
Can α-GalCer be stored when diluted to 200 ug/mL?
Storing α-GalCer diluted to 200 μg/mL is not recommended, as reactivity has been shown to decrease
What is the shelf life of CD1d tetramer?
CD1d tetramers are stable for at least 12 months when properly stored, and should be discarded when increased background or other signs of deterioration are noted.
What percent positive is expected for CD1d?
In a study of 20 healthy donors, Gumperz et al. reported a CD1d-positive range of undetectable (<0.01%) to 2.34% of CD3+ cells, with a median of 0.034% and a mean of 0.194%, when the tetramer was loaded with α-GalCer (J. Exp. Med. 2002; 195: 625). In a comparison of various mouse strains, Hammond et al. showed CD1d tetramer-positive cells varied depending on the tissue source, ranging from 0.5-1.4% in spleen/lymph node samples and higher (≥20%) in liver (J. Immunol.2001; 167:1164-1173).
What are MHC monomers?
An MHC monomer is a sub-component of an MHC tetramer, made up of the MHC complex (α-chain plus β-2 microglobulin for class I or α-chain plus β-chain for class II) folded together with a specific peptide. Biotinylated monomers are available for most associated tetramer products.
What are MHC monomers used for?
Investigators often select MHC monomers to create tetramers in their own labs with alternate labels or to generate smaller tetramer batches. Others use them for screening or immunization strategies. The production of metal-labeled tetramers for use with CyTOF instruments using MBLI monomers is becoming increasingly popular. A poster entitled “Metal-conjugated neutravidin for MHC multimer assays using mass cytometry” presented at Immunology 2015, May 8-12, 2015 in New Orleans by Ornatsky et al. can be found here.
What is the minimum purity of the peptides needed for use with the QuickSwitch™ kit?
The purity should be > 90%.
I’m using the same peptide as before, why is my exchange rate not as high?
Your peptide may contain cysteines. This means that if they are too old or have been exposed long to oxygen, they will have the propensity to make internal or inter peptide disulfide bonds. It is crucial to work (if possible) with recently made peptides to avoid these modifications.
Please contact our technical support team for further support regarding your unique experiments.